Abstract
Coronavirus disease 2019 (COVID-19), a disease caused by the novel betacoronavirus (SARS-CoV-2) has become a global pandemic threat. COVID-19 caused by SARS-CoV-2 is reported to originate in December 2019 in Wuhan, China and spreading rapidly around world. SARS-CoV-2 is structurally similar to the other coronaviruses, causing the severe respiratory syndrome (SARS-CoV) and the middle east respiratory syndrome (MERS-CoV), both binding to the angiotensin-converting enzyme 2 (ACE2) receptor to enter human cells. ACE 2 is widely expressed in several cells including, neural tissue. COVID-19 presents with fever and respiratory symptoms, possibly leading to acute respiratory distress (ARDS) but there are several published reports of acute cerebrovascular diseases, seizures, olfactory and gustatory dysfunctions, isolated involvement of cranial nerves, myositis/rabdhomyolisis as well myasthenic crisis (MC) and Guillain–Barré syndrome (GBS). The ARDS described during COVID-19 pandemic, coupled with respiratory muscle failure occurring in myasthenia gravis (MG), may result in a life-threatening condition, challenging for intensivists, pulmonologists and neurologists. Infections are recognized trigger of exacerbations and crisis in MG and patients with MG probably exhibit a mortality higher than the general population during this COVID-19 pandemic. We review the current state of knowledge on MG during the COVID-19 pandemic to focus the immunological and respiratory interplay between these two conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myasthenia gravis (MG) is a chronic autoimmune disorder characterized by fluctuating muscle weakness affecting ocular, bulbar and limb skeletal muscle groups due to a reduction of functional skeletal muscle nicotinic acetylcholine receptors (AChR) in 73–88% of patients and to structural alterations of the endplate as the effect of functionally related molecules at the neuromuscular junction caused by different autoantibodies [1,2,3,4,5,6,7]. Most AChR autoantibodies are polyclonal IgG, predominantly of the complement-activating subclasses IgG1 and IgG3, binding to the main immunogenic region (MIR) [6]. The onset of MG has been related to several factors including infections as trigger, like Hepatitis B and C, herpes simplex, HIV, and recently West Nile and Zika virus. However, no virus or other pathogens has been proven to have a specific link to MG [2, 6]. Epstein–Barr virus has been for many years a major candidate for induction of autoimmune disorders, due to its capacity to promote abnormal activation and survival of B-lymphocytes [4,5,6].
Corona Virus Disease 2019 (COVID-19) is a new illness, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that originate in Whuan, China. Symptoms are variable, ranging from mild to severe pneumonia, leading to acute respiratory distress syndrome (ARDS) and death in many cases [8,9,10,11,12,13,14,15,16,17,18,19]. Severe COVID-19 disease may be a risk factor in MG for many reasons, including an immunocompromised state related to baseline therapies and to respiratory muscle weakness [2, 3, 7]. According to the published literature, there is considerable variability in how MG patients respond to SARS-CoV-2 infection and it is unclear whether this virus can trigger myasthenic crisis (MC) and thus contributing to the development of an acute respiratory failure. In addition, some drugs used in therapeutic trials early in the pandemic, i.e., hydroxychloroquine and azithromycin could induce MG exacerbations [2, 14, 20, 21]. Several MG patients with SARS-CoV-2 infection have been recently described [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Here, we review the current state of knowledge on MG during the COVID-19 era and we discuss the immunological and respiratory interplay between MG and this new infection.
Overview on immunological and clinical manifestations of MG
Clinical and immunological subtypes of MG
Disease distribution and severity of MG is categorized according to the Myasthenia Gravis Foundation of America classification (MGFA) as ocular (OMG, class 1), or else mild (class II A and IIB), moderate (class IIIA and IIIB), severe (class IVA, IVB, and V) and finally as generalized MG (GMG). OMG (10–20% of patients) exclusively affects the outer ocular muscles and presents with ptosis and diplopia which can be transient, fluctuating or progressive during the day. GMG is defined as any clinical involvement of muscle groups other than outer ocular muscle, independently of the severity [1, 4, 6]. In respect of age at onset, MG is defined as early (onset at age lower than 45 or 50 or 60 years, i.e., EOMG) and late, if above 45 or 50 or 60, according to authors, i.e., (LOMG) [4, 6]. About 10–15% of all MG patients do have a thymoma-associated MG (TAMG); conversely, MG is present in approximately 30–45% of thymomas. Thymomas are slow-growing, locally invasive neoplasms of thymic epithelial cells (TECs) usually with mixed cortical and medullary properties, classified in types (A, AB, B1, B2, B3) depending on lymphocyte content and epithelial cell features [6]. Furthermore, MG patients are grouped according to their antibody status as AChR-antibody positive, muscle specific tyrosine kinase (MuSK) antibody positive and double sero-negative (SNMG, i.e., AChR and MuSK-antibody negative) [4, 6]. As immunopathogenetic mechanism, the auto-antibodies to AChR block the receptor, reducing the number of available receptors in the membrane; in this condition the activation of complement cascade leads to the destruction of endplate architecture with a widened synaptic cleft at the endplate [6]. In about 50% of patients with OMG and in at least 10–15% with GMG, where the testing for autoantibodies gives negative results, the myasthenic weakness comes from autoimmune processes directed to postsynaptic targets distinct from AChR [6]. There are several clinically important differences between anti-AChR antibody-positive and MuSK-positive MG; indeed, the latter subtype exhibits prominent oropharyngeal, facial, tongue, neck and respiratory muscle weakness [6].
The thymus in MG
The thymus is the primary source of Tregs, which constitute approximately 5–10% of the peripheral CD4+ T cell population and has a crucial role in the maintenance of immune homeostasis against self-antigens [4, 6]. The thymus plays a fundamental role in the pathogenesis of MG. Moreover, the thymus exhibits pathological changes in the majority of patients with AChR antibodies, whereas MuSK-MG so far is not associated with thymic abnormalities. In about 70% of patients with EOMG there are lymphofollicular hyperplasia with lymphoid follicles and germinal centres (GCs) within the thymus [6]. On the contrary, patients with LOMG show involution and atrophy of the thymus, where the lympho-epithelial tissue is gradually replaced by fat. Indeed, LOMG patients show similarities with TAMG regarding some autoantibodies, including those against voltage-gated Ca + and K + channels and titin present in 70% of patients aged over 60 years.
Myasthenic crisis (MC) and exacerbations
MC is a life-threatening manifestation of MG, associated with severe bulbar dysfunction and/or respiratory failure that in most cases requires mechanical ventilation (MV) and ICU admission [2, 3, 7, 21, 24,25,26,27,28, 32, 34]. Until the early 1960s, mortality related to MC was about 40% of cases. Today, the reports on mortality are heterogeneous and usually range between 5 and 12%, but higher rates up to 30% have also been recently described during the COVID-19 pandemic [32]. Impending MC, instead, defines a clinical condition that precedes MC (days to weeks) and also requires intermediate ICU admission [1,2,3,4, 7, 21, 24, 27, 32, 34]. Hallmarks of therapy for MC are symptomatic treatment with acetylcholinesterase inhibitors, plasmapheresis (PE) or polyvalent immunoglobulins (IVIG) as well as early start of steroids and immunosuppressants [21, 27, 28, 34].. PE, IVIG or a combination of both are the preferred treatments [2, 3, 6, 7]. MC mimic may develop in cases of excessive, acute or chronic overdose of anticholinesterase medications [3, 6, 21].
Interplay between MG and COVID-19
At the time of this writing, several patients with MG associated with SARS-CoV-2 infection have been reported in the English literature [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Main clinical and immunological features of the cases described are listed in Table 1.Camelo–Filho et al. [32] published the largest series of patients, noting that most cases had a severe course, requiring ICU admission in 87%, and MV in 73%, while 30% died.
Potential role of SARS-Cov-2-induced autoimmunity in MG
Infections, either microbial or viral, are major external causal factors in nearly all autoimmune disorders and in MG [2, 3, 7]. The simplest explanations would be that the impaired immunity results in an increased risk of infection; on the other hand, the infections will induce a polyclonal activation of immunoreactive cells that can include autoreactive B- and T-lymphocytes [2, 3]. Viral infections might trigger autoimmunity via several pathways, including molecular mimicry, epitope spreading, bystander activation, enhanced T-cell signaling and up-regulation of a series of cytokines and costimulatory molecules and/or immortalization of infected B- cells [2, 6, 7]. Such mechanisms are hypothesized, but not proven for MG etiology and still virus infection as the initial event in MG pathogenesis represents a putative model, perhaps with a local infection in the thymus [2].
The cytokine storm associated with a “dysregulated” immune response represents a main pathogenetic mechanisms of severe COVID-19 disease, which encompasses fever, coughing, dyspnea and pneumonia with respiratory failure [10,11,12, 14]. SARS-CoV-2 may result in severe lymphopenia, especially T-cell loss from CD26 T-cells apoptosis [11, 14].
Thymus is essential in the AChR-MG pathogenesis; moreover, thymus activity and T-lymphocyte functions are assumed to be involved in the protection against SARS-CoV-2 in children through the replacement of the T-cells destroyed by the apoptosis caused by the virus [35]. Interestingly, the thymus of children can prevent the inflammatory damage triggered by SARS-CoV-2 by an immunomodulating effect through the activity of regulatory T-cells (Treg), a specialized subset of CD4+T-cells active during the early periods of life, with a precise role in immunomodulation [35]. The loss of Treg function by age results in difficulty with the control of the immune responses and increased inflammation [35].
Some recent reports suggest that COVID-19 could trigger, sustain and perpetuate autoimmune conditions with various mechanisms which include molecular mimicry and bystander activation [36].
According to Lucchese et al. [36], SARS-CoV-2 viral proteome is sharing six amino acids sequences with three proteins, namely DAB1, AIFM, and SURF1 present in the human brainstem respiratory pace-maker neurons. Therefore, the immunological targeting of DAB1, AIFM1, and SURF1 might contribute to brainstem-related respiratory failure in COVID-19 patients [36]. To test this hypothesis, Lucchese et al. [36] proposed sera and cerebrospinal fluid from COVID-19 patients suffering from respiratory distress and/or neurological symptoms to be examined for immunoreactivity against the shared protein sequences. A part from the molecular mimicry mechanism as proposed by Lucchese et al. [36], the contribution of T-cells dysregulation in promoting autoantibody-producing B-cells could also play a role in enhancing autoimmunity triggered by SARS-CoV-2 [14, 18].
T-cells dysregulation in MG and COVID-19 at the crossroad
An imbalance between inflammatory T-helper 17 (Th-17) cells and Treg cells has been considered in chronic inflammatory autoimmune diseases, either systemic or organ-specific [18]. It is known that T reg cells play a key role in maintaining self-tolerance and regulating immune response, predominantly by suppressing effector T-cells. Interestingly, a characteristic immunological feature in patients with autoimmune diseases, including MG, is that Tregs are present in reduced numbers and/or have compromised functions, suggesting that an autoimmune condition could be associated with defective Tregs [37]. In experimental autoimmune MG, as reported by Liu et al. [37], the expanded Treg potently suppressed autoreactive T-and B-cell responses and attenuated the muscular weakness, reducing the circulating levels of AChR autoantibodies [37]. Moreover, the elegant study of Thiruppathy et al. [38] demonstrated that Treg-mediated suppression of effector T-cells was impaired in MG patients. The suppression of both polyclonal and AChR-activated T-cells from MG patients could be restored using Tregs isolated from healthy controls, revealing a potential novel therapeutic target [38].
In severely ill COVID-19 patients, there is a significant reduction in the level of peripheral Tregs, compared to mildly affected patients [39] and in bronchoalveolar lavage of the more severe SARS-CoV-2 infected patients, the IL-2 transcript, a cytokine that plays a major role in the generation and maintenance of Tregs in vivo, was reduced [40]. Furthermore, critically ill SARS-CoV-2 patients show increased levels of soluble IL-2R (CD25) that could interfere with IL-2 bioavailability, further promoting Tregs apoptosis [41]. Recently, Gladstone et al. [42] described a marked improvement in two patients with COVID-19/ARDS treated with administration of allogenic Tregs, derived from cord blood through intermittent intravenous infusions. Taken together these immunological data, although “the hyper immune-inflammation” underlying COVID-19 may be heterogeneous, in a subset of SARS-CoV-2 patients Tregs/Th-17 dysregulations could contribute to the enhancement of an autoimmune disease in a predisposed host [41]
There is growing evidence of IL-17 involvement in pathophysiology of MG. Animals with experimentally acquired MG demonstrate upregulation of IL-17, which is the most well-known member of a multifunctional cytokine family produced by Th-17 cells [43]. In the context of an inflammatory cytokine milieu, IL-17 exerts various biologic activities, such as enhancing Th1 responses, promoting cytotoxic T-cell activity, as well as neutrophil migration and activation during viral infection, but also regulation of B- cells functions and GCs development [38, 39, 43]. In females with severe EOMG without thymoma but with thymic hyperplasia, the IL-17A plasma concentration was significantly higher than in control healthy subjects, suggesting a role in the pathogenesis of this subgroup of patients [43]. Furthermore, in an animal model of experimental autoimmune MG, IL-17 knockout mice had no evidence of weakness, low levels of AChR antibodies and retention of AChR at the neuromuscular junction [44]. Xie et al. [43] found that patients with purely OMG were more likely to develop generalized weakness with higher IL-17A levels. Given that, all the data clearly suggest an imbalance between Treg cells (dysfunction) and Th-17 cells (hyperactivity) in the promotion and/or amplification of immune-inflammation underlying AChR autoantibodies MG.
The clinical disease severity of MERS-CoV, SARS-CoV and SARS-CoV-2 has been correlated to Th-17 cytokine levels [45, 46]. Furthermore, in COVID-19 infection developing ARDS, IL-17 plays a critical role in driving the production of proinflammatory cytokines, the induction of granulocyte colony-stimulating factor expression, and the trigger of neutrophil accumulation in lung tissue, leading to diffuse alveolar damage [47]. IL-17 inhibition has been previously adopted as successful strategy to reduce the injury associated with inflammatory autoimmune diseases, including psoriasis and psoriatic arthritis [45]. In patients with MG, COVID-19 infection could amplify Tregs/IL-17 imbalance and induce an increase in the level of circulating anti-AChR antibodies, possibly related the development of MC. Conceivably a Tregs therapy (adoptive or in vivo Treg expansion) and therapies inhibiting IL-17 (secukinumab, ixekizumab) and his receptor (brodalumab) could play a potential therapeutic role in patients with severe SARS-CoV-2 and MG. Figure 1 summarizes the speculative autoimmune mechanisms due to SARS-CoV-2 infection in patients with MG.
Hypothetical mechanisms by which SARS-CoV-2 could trigger and amplify autoimmunity in MG. SARS-CoV-2 shares amino acids sequences with host components and results in the cross-activation of autoreactive T o B cells promoting autoimmune manifestation during COVID-19 (molecular mimicry). In SARS-CoV-2 infection, antigen-presenting cells (APCs) process and present viral antigens for the recognition by T cells, which are stimulated to produce proinflammatory cytokines (TGF-b, IL-6, IL-23) with Th-17 differentiation. Th-17 cells secrete IL-17 that promotes neutrophils recruitment in the lung, resulting in diffuse alveolar damage and release of self-antigens, and induces the differentiation of B cells into plasma cells. Subsequently, the autoimmune response can be amplified by the mechanisms of epitope spreading and bystander activation. Furthermore, in severe COVID-19, increased level of soluble IL-2R (CD25) could interfere with IL-2 bioavailability, resulting in Treg dysfunction. Finally, Treg/TH-17 imbalance might amplify the production of autoantibodies against AChR in patients with MG affected by COVID-19.
Respiratory failure in SARS-CoV-2 and MG
Physiological interplay between COVID-19 and M G
About 20% of patients infected by SARS-CoV-2 develop acute respiratory failure and require ICU admission [48]. Lung physiology in COVID-19 associated ARDS is very complex and heterogeneous. Patients with SARS-CoV-2-related respiratory failure exhibit different clinical-physiological phenotypes, depending on many factors including the interaction between the virus and the host, the patient comorbidities, and the time elapsed between the onset of the disease and the admission to the hospital.
Gattinoni et al. [49] suggested an L phenotype of ARDS, which differs from the classic ARDS by presenting a marked hypoxemia with preserved compliance and limited ground-glass densities on computed tomography (CT) scan and an H phenotype that fulfills the ARDS criteria. In L phenotype, the intravascular pathology (i.e., loss of regulation of perfusion, microcirculatory clot formation) may cause hypoxemia by increasing dead space and worsening ventilation-perfusion ratio. However, a recent study by Grasselli et al. [50] provided evidence that patients with COVID-19 in MV have a form of injury similar to that of patients with a classical ARDS unrelated to Covid-19. Notably, patients with COVID-19-related ARDS who show a reduction in respiratory system compliance together with increased D-dimer concentrations have high mortality rates [50].
The complex physiological interplay between SARS-CoV-2 infection and MG makes the management of patients in ICU quite challenging. Indeed, in patients with SARS-CoV-2 infection and MG admitted to ICU, the host-virus interaction can generate in our experience three different scenarios: (1) MC without radiographic features of ARDS; (2) COVID-19 pneumonia compatible with ARDS criteria, but without evidence of respiratory muscle dysfunction due to MC; (3) ARDS combined with MC.
Respiratory muscles assessment and diaphragm fatigue in MG with SARS-CoV-2 infection
In patients with exacerbations of MG, a crucial issue in ICU is always the decision on timing of MV and the assessment of respiratory muscle function by static lung volume and pressure measurements. In spontaneous breathing, in patients with bulbar dysfunction or with acute respiratory failure a reliable non-invasive respiratory muscle assessment in ICU is challenging. Dynamic respiratory test (i.e., maximal voluntary ventilation) cannot be performed in cases of acute respiratory failure, but it best reveals a “myasthenic pattern” with decremental reduction of inspiratory and expiratory volumes with each breath [51]. Usually, the most frequently noticed abnormality in lung volumes of patients with respiratory muscle weakness is a reduction in vital capacity (VC); however, in patients with MG admitted to ICU, repeated measurements of VC cannot predict MV requirement, due to the erratic nature of the disease [52]. Despite the difficult monitoring of respiratory muscles function, it is widely accepted that patients displaying VC < 20 mL/kg or negative inspiratory force (NIF) < − 20 cmH2O require ventilator support [7, 52].
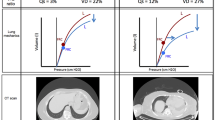
The diaphragmatic fatigability makes patients with SARS-CoV-2 infection and MG more prone to develop respiratory muscles fatigue, requiring immediate intubation and MV. The physiological framework underlying the concept of diaphragmatic fatigue has been outlined by Bellemare and Grassino more than 20 years ago, who established that above a certain load threshold the diaphragm is no longer able to support its work [56]. In most cases, COVID-19 is associated with strong activation of the respiratory drive, that results in a vigorous inspiratory effort (elevated Pdi and Pes swing); thus, MG patients might be subject to excessive respiratory muscles load [57, 58]. However, in this condition, some physiological adaptation mechanisms are activated to avoid fatigue, mainly by central neural output inhibition of the respiratory muscles leading in acute hypoventilation and hypercapnia [59, 60]. As a consequence of such mechanisms, the superimposition of SARS-CoV-2 infection in MG might act as a physiological trigger of diaphragmatic task failure. Figure 2 summarizes the possible physiological events in MG in the course of SARS-CoV-2 pneumonia.
Respiratory physiological interplay between SARS-CoV-2 infection and MG. MG patients with COVID-19 pneumonia are subject to excessive mechanical load due to central activation (Panel A). The pressure (Pdi) that the diaphragm is able to generate to maintain adequate VT gradually decreases over time showing a “myasthenic pattern” (Panel B). At this point, despite the activation of the respiratory drive, microatelectasis appears due to the weakness of respiratory muscles, and dynamic compliance is further reduced increasing the work of breathing. Subsequently, when respiratory muscles approach the fatigue threshold (TTdi > 0.15), acute hypoventilation and hypercapnia result from central neural output inhibition of the diaphragm, and patients require immediate mechanical ventilation (Panel C). TTdi: diaphragmatic tension-time integral, VE: minute ventilation, Pd: transdiaphragmatic pressure, Pes: esophageal pressure.
Interestingly, in a subgroup of SARS-CoV-2 infected patients there is a disconnection between severe hypoxemia and relatively mild respiratory discomfort; this condition is named as silent or “happy” hypoxemia, characterized by poor activation of respiratory drive and reduced ventilatory response, despite the severe hypoxemia [61, 62]. MG patients with SARS-CoV-2 infection exhibiting the physiological features of “happy hypoxemia”, being subjected to a lower load on the respiratory muscles, could be conceivably less prone to develop MC.
Mechanical ventilation
The decision whether or not intubate a patient with severe SARS-CoV-2 depends on a number of factors [63]. Signs of unsustainable work of breathing, refractory hypoxemia, hypercapnia or acidemia, inadequate airway protection, and altered consciousness are all possible clinical indications for endotracheal intubation [63]. Present guidelines recommend that clinicians should follow the ventilatory protective strategy developed during the past two decades for classical ARDS [64, 65]. Intensivists should set the ventilator to deliver a tidal volume (VT) of 6 ml of predicted body weight or less, and the plateau pressure should not exceed 30 cmH2O to avoid the development of ventilator lung injury.
Also in patients with MG, the need for tracheal intubation depends on clinical judgment, and the evaluation of respiratory muscles function is crucial to decide on early intubation [3, 66, 67] Furthermore, in cases of upper airway or bulbar weakness due to MC, the oropharyngeal collapse might increase the work of breathing and exposes the patient to aspiration risk; therefore, it may be a condition requiring elective intubation. After intubation, excess secretions and diaphragmatic dysfunction could lead to atelectasis in dependent areas of the lung, with an increase in intrapulmonary shunt and a reduction in compliance of the respiratory system. Aggressive chest physiotherapy, airway clearance with frequent suctioning, and daily spontaneous breathing trial should be implemented to facilitate weaning from MV [66, 67]. Indeed, in patients with MC, once intubated, weaning from mechanical ventilation is challenging, and extubation fails in about 30% of cases [3, 68].
Non-Invasive ventilation (NIV)
The role of non-invasive ventilation (NIV) during SARS-CoV-2 pandemic is debated due to major concerns about using bio-aerosol producing techniques and risk of staff contamination [20, 69, 70]. Furhermore, most data about NIV application during the pandemic derived from non-randomized trials [69, 70]. In pre-COVID-19 era, ATS/ERS Guidelines suggested caution in using NIV in de novo respiratory failure due to the risk of worsening patient outcome with delayed intubation, and made no recommendation for or against the use of NIV during pandemics [71].
In patients with MG exacerbations or MC during SARS-CoV-2 infection, a trial of NIV before endotracheal intubation can be indicated, especially if there are no radiographic changes compatible with ARDS. However, NIV must be used with extreme caution in presence of severe bulbar dysfunction at risk of aspiration, and tracheal intubation is often the most rational choice. Moreover, NIV may reduce the need for tracheal intubation in selected patients with MC, shortening the duration of ventilation and length of stay in ICU [72, 73]. In a recent German multicenter retrospective study, out of 92 patients with MC who received ventilation, NIV was successful in 38% and it was associated with shorter duration of ventilation and ICU stay [3]. Interestingly, in the Neumann et al. study [3], the NIV trial did not extend the duration of MV in patients who failed NIV and who were subsequently intubated as compared to patients without NIV. So NIV might be considered in appropriate patients before intubation [3]. Taken together all these data, in MG patients and SARS-CoV-2 infection, the development of bulbar dysfunction or the ARDS criteria could require in most cases an early intubation.
Conclusions
Clinical responses of MG patients during SARS-CoV-2 infection are unpredictable and challenging for clinicians. From the pathogenetic point of view, it has been hypothesized that Treg/Th17 imbalance in the course of SARS-CoV-2 could amplify or trigger the excessive autoimmune response. Though there is no direct evidence for the involvement of proinflammatory cytokines and chemokines in lung pathology, the change of laboratory parameters, including elevated serum cytokine, chemokine level in infected patients correlating with the severity of the disease and adverse outcome, confirmed a possible role for hyper-inflammatory responses in COVID-19 [41].
In a setting of MC associated with SARS-COV-2 infection, the respiratory muscle function evaluation is crucial in deciding the endotracheal intubation timing. A NIV trial can be indicated and can avoid endotracheal intubation. Moreover, in some clinical conditions such as in cases of excessive work of breathing, the development of bulbar dysfunction or ARDS, early intubation and MV could be required.
The current state of knowledge on the interaction between MG and COVID-19 is changing rapidly due to growing experience in patients. The ongoing international registry launched by Muppidi et al. will surely provide informations useful for the care of MG patients during this pandemic [74,75,76].
References
Jaretzki A 3rd, Barohn RJ, Ernstoff RM et al (2000) Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 55(1):16–23. https://doi.org/10.1212/wnl.55.1.16
Gummi RR, Kukulka NA, Deroche CB et al (2019) Factors associated with acute exacerbations o myasthenia gravis. Muscle Nerve 60:693–699. https://doi.org/10.1002/mus.26689
Neumann B, Angstwurm K, Mergenthaler P et al (2020) Myasthenic crisis demanding mechanical ventilation: a multicenter analysis of 250 cases. Neurology 94:e299–e313. https://doi.org/10.1212/WNL.0000000000008688
Mantegazza R, Bernasconi P, Cavalcante P (2018) Myasthenia gravis: from autoantibodies to therapy. Curr Opin Neurol 31:517–525. https://doi.org/10.1097/WCO.0000000000000596
Leis A, Szatmary G, Ross MA et al (2014) West Nile virus infection and myasthenia gravis. Muscle Nerve 49:26–29. https://doi.org/10.1002/mus.23869
Melzer N, Ruck T, Gold R et al (2016) Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J Neurol 263:1473–1494. https://doi.org/10.1007/s00415-016-8045-z
Roper J, Fleming ME, Long B et al (2017) Myasthenia gravis and crisis: evaluation and management in the emergency department. J Emerg Med 53:843–853. https://doi.org/10.1016/j.jemermed.2017.06.009
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Guan WJ, Ni ZY, Hu Y et al (2020) Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med 382:1708–1720. https://doi.org/10.1056/NEJMoa2002032
Chen G, Wu D, Guo W, Cao Y et al (2020) Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130:2620–2629. https://doi.org/10.1172/JCI137244
Wu C, Chen X, Cai Y et al (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China JAMA Intern Med 180:934–943. https://doi.org/10.1001/jamainternmed.2020.0994
Zhang J, Wang X, Jia X et al (2020) Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan. China Clin Microbiol Infect 26:767–772. https://doi.org/10.1016/j.cmi.2020.04.012
Holshue ML, DeBolt C, Lindquist S et al (2020) First case of 2019 Novel Coronavirus in the United States. N Engl J Med 382:929–936. https://doi.org/10.1056/NEJMoa2001191
Mao L, Jin H, Wang M et al (2020) Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 77:683–690. https://doi.org/10.1001/jamaneurol.2020.1127
Asadi-Pooya AA, Simani L (2020) Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci 413:116832. https://doi.org/10.1016/j.jns.2020.116832
Roe K (2020) Explanation for COVID-19 infection neurological damage and reactivations. Transbound Emerg Dis 67:1414–1415. https://doi.org/10.1111/tbed.13594
Yashavantha Rao HC, Jayabaskaran C (2020) The emergence of a novel coronavirus (SARS-CoV-2) disease and their neuroinvasive propensity may affect in COVID-19 patients. J Med Virol 92:786–790. https://doi.org/10.1002/jmv.25918
Cao Y, Amezquita RA, Kleinstein SH et al (2016) Autoreactive T cells from patients with myasthenia gravis are characterized by elevated IL-17, IFN-g, and GM-CSF and diminished IL-10 production. J Immunol 196:2075–2084. https://doi.org/10.4049/jimmunol.1501339
Diaz JH (2020) Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med 27(3):taaa041. https://doi.org/10.1093/jtm/taaa041
Anand P, Slama MCC, Kaku M et al (2020) COVID-19 in patients with myasthenia gravis. Muscle Nerve 62(2):254–258. https://doi.org/10.1002/mus.26918
Delly F, Syed MJ, Lisak RP et al (2020) Myasthenic crisis in COVID -19. J Neurol Sci 414:116888. https://doi.org/10.1016/j.jns.2020.116888
Huber M, Rogozinski S, Puppe W et al (2020) Postinfectious onset of myasthenia gravis in a COVID-19 patient. Front Neurol 11:576153. https://doi.org/10.3389/fneur.2020.576153
Rein N, Haham N, Orenbuch-Harroch E et al (2020) Description of 3 patients with myasthenia gravis and COVID-19. J Neurol Sci 417:117053. https://doi.org/10.1016/j.jns.2020.117053
Hübers A, Lascano AM, Lalive PH et al (2020) (2020) Management of patients with generalised myasthenia gravis and COVID-19: four case reports. J Neurol Neurosurg Psychiatry 91:1124–1125. https://doi.org/10.1136/jnnp-2020-323565
Singh S, Govindarajan R (2020) COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg 196:106045. https://doi.org/10.1016/j.clineuro.2020.106045
Kushlaf H (2020) COVID-19 in muscle-specific kinase myasthenia gravis: a case report. Muscle Nerve 62(4):E65–E66. https://doi.org/10.1002/mus.27020
Restivo DA, Centonze D, Alesina A et al (2020) Myasthenia gravis associated with SARS-CoV-2 infection. Ann Intern Med 10:L20-0845. https://doi.org/10.7326/L20-0845
Salik I, Rodhouse HB, Barst S (2020) Myasthenic crisis in the setting of Coronavirus Disease 2019 (COVID-19). J Clin Anesth 67:110001. https://doi.org/10.1016/j.jclinane.2020.110001
Ramaswamy SB, Govindarajan R (2020) COVID-19 in refractory myasthenia gravis- a case report of successful outcome. J Neuromuscul Dis 7:361–364. https://doi.org/10.3233/JND-200520
Scopelliti G, Osio M, Arquati M et al (2020) Respiratory dysfunction as first presentation of myasthenia gravis misdiagnosed as COVID-19. Neurol Sci 41(12):3419–3421. https://doi.org/10.1007/s10072-020-04826-3
Sriwastava S, Tandon M, Kataria S et al (2020) New onset of ocular myasthenia gravis in a patient with COVID-19: a novel case report and literature review. J Neurol 12:1–7. https://doi.org/10.1007/s00415-020-10263-1
Camelo-Filho AE, Silva AMS, Estephan EP et al (2020) Myasthenia Gravis and COVID-19: clinical characteristics and outcomes. Front Neurol 11:1053. https://doi.org/10.3389/fneur.2020.01053
Aksoy E, Oztutgan T (2020) COVID-19 presentation in association with myasthenia gravis: a case report and review of the literature. Case Rep Infect Dis 20:1–4. https://doi.org/10.1155/2020/8845844
Moschella P, Roth P (2020) Isolated COVID-19 infection precipitates myasthenia gravis crisis : a case report. Clin Pract Cases Emerg Med 4:524–526. https://doi.org/10.5811/cpcem.2020.9.49049
Güneş H, Dinçer S, Acıpayam C et al (2020) What chances do children have against COVID-19? Is the answer hidden within the thymus? Eur J Pediatr 13:1–4. https://doi.org/10.1007/s00431-020-03841-y
Lucchese G, Flöel A (2020) Molecular mimicry between SARS-CoV-2 and respiratory pacemaker neurons. Autoimmun Rev 19(7):102556. https://doi.org/10.1016/j.autrev.2020.102556
Liu R, Zhou Q, La Cava A et al (2010) Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur J Immunol 40(6):1577–1589. https://doi.org/10.1002/eji.200939792
Thiruppathi M, Rowin J, Li Jiang Q et al (2012) Impaired regulatory function in circulating CD4(+) CD25(high) CD127(low/-) T cells in patients with myasthenia gravis. Clin Immunol 145:209–223. https://doi.org/10.1016/j.clim.2012.09.012
Wang W, Su B, Pang L et al (2020) High-dimensional immune profiling by mass cytometry revealed immunosuppression and dysfunction of immunity in COVID-19 patients. Cell Mol Immunol 17:650–652. https://doi.org/10.1038/s41423-020-0447-2
Kalfaoglu B, Almeida-Santos J, Tye CA et al (2020) T-cell dysregulation in COVID-19. Biochem Biophys Res Commun 7:S0006. https://doi.org/10.1016/j.bbrc.2020.10.079
Quin C, Zhou L, Hu Z et al (2020) Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan. China. Clin infect Dis 71(15):762–768. https://doi.org/10.1093/cid/ciaa248
Gladstone DE, Kim BS, Mooney K et al (2020) Regulatory T cells for treating patients with COVID-19 and acute respiratory distress syndrome: two case reports. Ann Intern Med 173(10):852–853. https://doi.org/10.7326/L20-0681
Xie Y, Hai-feng Li, Jiang B et al (2016) Elevated plasma interleukon-17A in a subgroup of Myasthenia Gravis patients. Cytokine 78:44–46. https://doi.org/10.1016/j.cyto.2015.06.011
Aguilo-Seara G, Xie Y, Sheehan J et al (2017) Ablation of IL-17 expression moderates experimental autoimmune myasthenia gravis disease severity. Cytokine 96:279–285. https://doi.org/10.1016/j.cyto.2017.05.008
Pacha O, Sallman MA, Evans SE et al (2020) COVID-19: a case for inhibiting IL-17? Nature reviews Immunol 20:345–346. https://doi.org/10.1038/s41577-020-0328-z
Chen G, Wu D, Guo W et al (2020) (2020) Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130:2620–2629. https://doi.org/10.1172/JCI137244
Muir R, Osbourn M, Dubois AV et al (2016) Innate lymphoid cells are the predominant source of IL-17A during the early pathogenesis of acute respiratory distress syndrome. Am J Respir Crit Care Med 193:407–416. https://doi.org/10.1164/rccm.201410-1782OC
Wang D, Hu B, Hu C et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323:1061–1069. https://doi.org/10.1001/jama.2020.1585
Gattinoni L, Chiumello D, Caironi P et al (2020) COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 46:1099–1102. https://doi.org/10.1007/s00134-020-06033-2
Grasselli G, Tonetti T, Protti A et al (2020) Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med 8(12):1201–1208. https://doi.org/10.1016/S2213-2600(20)30370-2
Heliopoulos I, Palakas G, Vadikolias K et al (2003) Maximal voluntary ventilation in myasthenia gravis. Muscle Nerve 27:715–719. https://doi.org/10.1002/mus.10378
Rieder P, Louis M, Jolliet P et al (1995) The repeated measurement of vital capacity is a poor predictor of the need for mechanical ventilation in myasthenia gravis. Intensive Care Med 21:663–668. https://doi.org/10.1007/BF01711545
Godoy DA, Vaz de Mello LJ, Masotti L et al (2013) The myasthenic patient in crisis: an update of the management in neurointensive care unit. Arq Neuropsiquiatr 71:627–639. https://doi.org/10.1590/0004-282X20130108
Lizarraga AA, Lizarraga KJ, Benatar M (2016) Getting rid of weakness in the ICU: an updated approach to the acute management of myasthenia gravis and Guillain-Barré syndrome. Semin Neurol 36:615–624. https://doi.org/10.1055/s-0036-1592106
American Thoracic Society/European Respiratory Society (2002) ATS/ERS Statement on respiratory muscle testing. Am J Respir crit Care Med 166(4):518–624. https://doi.org/10.1164/rccm.166.4.518
Bellemare F, Grassino A (1982) Effect of pressure and timing of contraction on human diaphragm fatigue. J Appl Physiol 53:1190–1195. https://doi.org/10.1152/jappl.1982.53.5.1190
Esnault P, Hraiech CMS, Cardinale M (2020) High respiratory drive and excessive respiratory efforts predict relapse of respiratory failure in critically ill patients with COVID-19. Am J Respir Crit Care Med 202:1173–1178. https://doi.org/10.1164/rccm.202005-1582LE
Spinelli E, Mauri T, Beitler JR et al (2020) Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med 46:606–618. https://doi.org/10.1007/s00134-020-05942-6
Garcia Rio F, Prados C, Díez Tejedor E et al (1994) Breathing pattern and central ventilator drive in mild and moderate generalized myasthenia gravis. Thorax 49(7):703–706. https://doi.org/10.1136/thx.49.7.703
Laghi F, Shaikh HS, Morales D et al (2014) Diaphragmatic neuromechanical coupling and mechanisms of hypercapnia during inspiratory loading. Respir Physiol Neurobiol 198:32–41. https://doi.org/10.1016/j.resp.2014.03.004
Dhont S, Derom E, Van Braeckel E et al (2020) The pathophysiology of”happy” hypoxemia in COVID-19. Respir Res 21:198. https://doi.org/10.1186/s12931-020-01462-5
Tobin MJ, Laghi F, Jubran A (2020) Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med 202:356–360. https://doi.org/10.1164/rccm.202006-2157CP
Berlin DA, Gulick RM, Martinez FJ (2020) Severe Covid-19. N Engl J Med 383:2451–2460. https://doi.org/10.1056/NEJMcp2009575
Alhazzani W, Moller MH, Arabi YM et al (2020) Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med 46:854–887. https://doi.org/10.1007/s00134-020-06022-5
Matthay MA, Aldrich JM, Gotts JE (2020) Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med 8:433–434. https://doi.org/10.1016/S2213-2600(20)30127-2
Sakaguchi H, Yamashita S, Hirano T et al (2012) Myasthenic crisis patients who require intensive care unit management. Muscle Nerve 46:440–442. https://doi.org/10.1002/mus.23445
Varelas PN, Chua HC, Natterman J et al (2002) Ventilatory care in myasthenia gravis crisis: assessing the baseline adverse event rate. Crit Care Med 30:2663–2668. https://doi.org/10.1097/00003246-200212000-00009
Rabinstein AA, Mueller-Kronast N et al (2005) Risk of estubation failure in patients with myasthenic crisis. Neurocrit Care 3:213–215. https://doi.org/10.1385/NCC:3:3:213
Yang X, Yu Y, Xu J et al (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Res Med 8(5):475–481. https://doi.org/10.1016/S2213-2600(20)30079-5
Franco C, Facciolongo N, Tonelli R et al (2020) Feasibility and clinical impact of out-of-ICU non-invasive respiratory support in patients with COVID-19 related pneumonia. Eur Respir J 56:2002130. https://doi.org/10.1183/13993003.02130-2020
Rochwerg B, Brochard L, Elliott MW et al (2017) Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 50:1602426. https://doi.org/10.1183/13993003.02426-2016
Rabinstein A, Wijdicks EF (2002) BiPAP in acute respiratory failure due to myasthenic crisis may prevent intubation. Neurology 59(10):1647–1649. https://doi.org/10.1212/01.wnl.0000033797.79530.16
Seneviratne J, Mandrekar J, Wijdicks EF et al (2008) Noninvasive ventilation in myasthenic crisis. Arch Neurol 65:54–58. https://doi.org/10.1001/archneurol.2007.1
Muppidi S, Guptill JT, Jacob S et al (2020) COVID-19 associated risk and effects in myasthenia gravis (CARE-MG). Lancet Neurol 19(12):970–971. https://doi.org/10.1016/S1474-4422(20)30413-0
International MG/COVID-19 Working Group;Jacob S , Muppidi S et al (2020) Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci 412:116803. https://doi.org/10.1016/j.jns.2020.116803
Hoang P, Hurtubise B, Muppidi S (2020) Clinical Reasoning : therapeutic considerations in myasthenic crisis due to COVID-19 infection. Neurology 95(18):840–843. https://doi.org/10.1212/WNL.0000000000010651
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Galassi, G., Marchioni, A. Myasthenia gravis at the crossroad of COVID-19: focus on immunological and respiratory interplay. Acta Neurol Belg 121, 633–642 (2021). https://doi.org/10.1007/s13760-021-01612-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-021-01612-6